Recently, Professor Zhiyong Jiang's team in key laboratory of innovation and transformation of natural medicine in Henan province has made the latest progress in the field of asymmetric De Mayo reaction catalyzed by visible light. The relevant results entitled "Asymmetric photoredox catalytic formal De Mayo reaction enabled by sensitization-initiated electron transfer" was published in the journal Nature Chemistry (DOI: 10.1038/s41557-024-01502-3).
De Mayo reaction, discovered by Professor De Mayo, provides an effective chemical synthesis method to construct 1, 5-dicarbonyl skeleton by the reaction of 1, 3-diketone with olefin which has been widely used in the synthesis of natural products. Due to the unregulated activation of the substrate 1, 3-dione in the classical De Mayo reaction, uncontrolled structural racemates are easily produced. The "Sensitized initiated electron transfer (SenIET)" strategy proposed by Konig's group utilizes visible light and photosensitizers to realize the free radical reactions in the photoredox cycle, breaking through the thermodynamic limitations of the classical De Mayo reaction, while the efficient and universal catalytic asymmetric De Mayo reaction has not been reported.
Professor Zhiyong Jiang's team performed the SenIET reaction strategy, using the reaction substrate enol as the electron donor, DPZ as the visible light photosensitizer, and bispyrene chiral phosphoric acid as the chiral catalyst and co-sensitizer, in the reaction of 2-pyridine carbonyl substrates and olefin substrates (cyclic and non-cyclic enamines, olethers, neutral olefins, and cyclic internal olefins) to obtain the structurally diverse chiral 1, 5-dione structures with excellent yield and enantioselectivity, and achieved the asymmetric photoredox De Mayo reaction, solving the stereoselectivity problem in this reaction (Fig. 1). The research results of Professor Jiang's team developed the visible light asymmetric catalytic method in De Mayo reaction, and provided a solution for the efficient synthesis of 1, 5-dicarbonyl skeletons and natural active products.
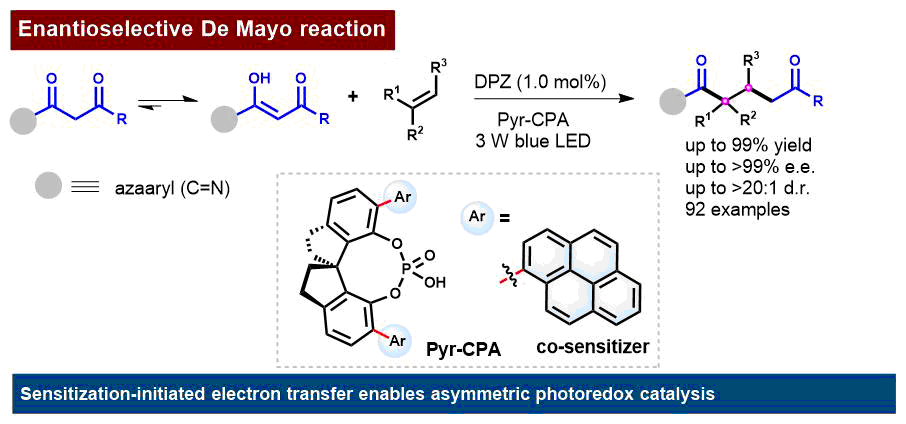
Figure 1 Asymmetric photoredox De Mayo reaction
Xin Sun, PhD student of Grade 2022, is the first author, and Professor Zhiyong Jiang is the corresponding author. Henan University was listed the first unit and the research was supported by the National Natural Science Foundation and other projects.
Professor Zhiyong Jiang, Member of the Communist Party of China, Doctor, Professor, and a doctoral supervisor. His research interests are asymmetric organic catalysis and visible light asymmetric organic catalysis. He has published more than 120 academic papers in international journals including Nature Chemistry, Nature Communication, J. Am. Chem. Soc. and Angew. Chem. Int.Ed.. He has been selected as National Science Foundation for Distinguished Young Scholars of China, New Century Excellent Researcher Award Program from Ministry of Education of China, national candidates for the Millions of Talents Project, young and middle-aged experts with outstanding contributions to the country, State Council special allowance expert, provincial distinguished professor, provincial outstanding expert, and provincial outstanding professional and technical talent. He is also a member of the editorial board of SCI journals such as Organic Chemistry, a senior member of the Chinese Chemical Society, a member of the photochemistry Committee of the Chinese Chemical Society, a member of the Photochemistry and photobiology committee of the Chinese Photographic Society, and an executive director of the Provincial Chemical Society.
Original URL: https://www.nature.com/articles/s41557-024-01502-3
Resource: Henan Key Laboratory of Natural Medicine Innovation and Transformation

 Research /
Research Achievements /
Science & Technology /
School of Chemistry and Molecular Sciences /
Content
Research /
Research Achievements /
Science & Technology /
School of Chemistry and Molecular Sciences /
Content


