Nitrates are prevalent in industrial and agricultural waste and pose a serious threat as soil and water contaminants. Recent studies have focused on converting nitrate back into ammonia, realizing the “waste-to-wealth” conversion. Through the application of electrocatalysis, this nitrate reduction reaction process has proven highly effective, achieving close to 100% Faradaic efficiency. This efficiency offers also an alternative to the traditional ammonia synthesis required in the Haber-Bosch process. Currently, most researches are performed in neutral and basic media, while reduction in acidic media remains rare, attributed to the usually low stability of catalysts in acid as well as the significantly enhanced hydrogen evolution reaction under acidic conditions. This limits the direct application to a large number of industrial wastes originating from sectors like mining, metallurgy, or the fiber industry.
Cu-based nanomaterials are regarded as favorable catalysts for nitrate reduction reaction, attributed to their highly occupied but still open d-orbitals with similar energy levels to the LUMO π* of nitrate, which facilitates the simple transfer of electrons from metals to adsorbed nitrate, thus a preactivation for the consecutive electrochemical process. Moreover, Cu-based materials have a high overpotential for hydrogen evolution reaction, this opening the reductive potential window for nitrate reduction. Recently, research on Cu-based catalysts was focused on single atom catalysts, which in principle could enhance the ammonia yield rate per Cu greatly. However, these catalysts are unstable especially in acidic conditions causing leaching of metal ions. In summary, it is important to realize the precise formation of Cu-based catalysts, and keep their stability in acidic solution.
Recently, Prof. Wensheng Yang’s and Prof. Zhihong Tian’s groups have developed a new method to mediate the valence of Cu-based clusters, by using mild annealing conditions to facilitate a Fehling’s-like reaction, leading to the supporting of Cu-based clusters on the oxocarbon substrate (Figure 1). The synthesized Cu2O clusters exhibits a superior electrocatalytic activity and excellent stability for nitrate reduction under acidic electrolyte conditions, with a high yield rate of ammonia at 3.31 mmol h-1 mgcat-1 and a Faradaic efficiency of 92.5% achieved at a potential of -0.4 V (vs. RHE).
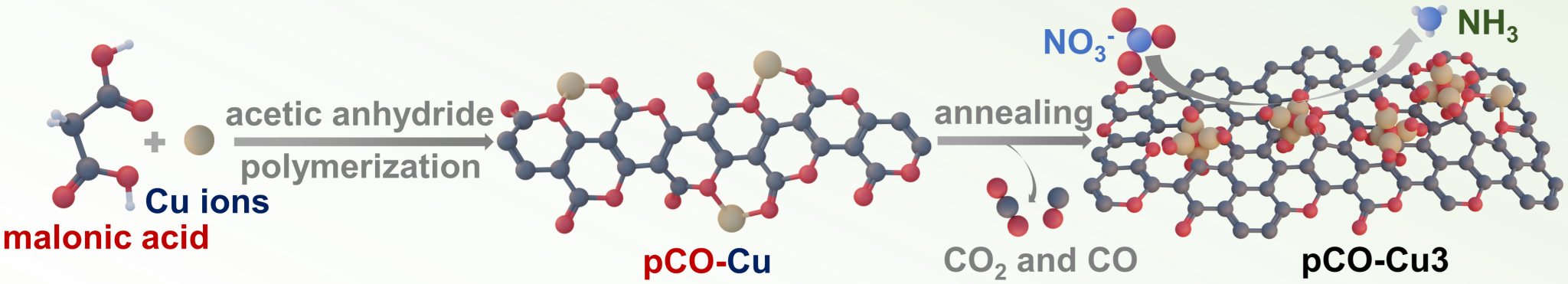
Figure 1. Schematic illustration of oxocarbon annealing procedure to mediate the formation of Cu-based clusters.
The relative achievements have been published on the journal Advanced Materials with the title Red Carbon Mediated Formation of Cu2O Clusters Dispersed on the Oxocarbon Framework by Fehling’s Route and their Use for the Nitrate Electroreduction in Acidic Conditions. Prof. Wensheng Yang and Prof. Zhihong Tian in the Engineering Research Center for Nanomaterials of Henan University are the co-corresponding authors. Dr. Jingwen Ba, a postdoctoral fellow in the Engineering Research Center for Nanomaterials of Henan University, and Dr. Hongliang Dong in the Center for High Pressure Science and Technology Advanced Research are the co-first authors. This research is supported by the National Natural Science Foundation of China, Henan University and Max Planck Society.
Link to the paper: https://onlinelibrary.wiley.com/doi/10.1002/adma.202400396
Resource:Engineering Research Center for Nanomaterials

 Research /
Research Achievements /
Science & Technology /
School of Chemistry and Molecular Sciences /
Content
Research /
Research Achievements /
Science & Technology /
School of Chemistry and Molecular Sciences /
Content


