Recently, Prof. JIANG Zhiyong’s research group published their new research achievement entitled Formal Enantioconvergent Substitution of Alkyl Halides via Catalytic Asymmetric Photoredox Radical−Radical Cross−Coupling in Nature Communications.
Prof. JIANG Zhiyong’s group has been committed to the research of asymmetric H-bonding catalysis and has made important progress in many aspects. Through establishing the visible light-driven photoredox catalysis system of non-metallic photosensitizer DPZ and the chiral phosphonic acid (CPA), they completed the substitution reaction in form of the N-aryl amino acids and the α-carbonyl halogenated hydrocarbons, synthesized α-tertiary and α-quaternary carbon amino chiral carbonyl compounds with high enantioselectivity and chemoselectivity, which provided a convenient and feasible method for the synthesis of chiral aminocarbonyl compounds.
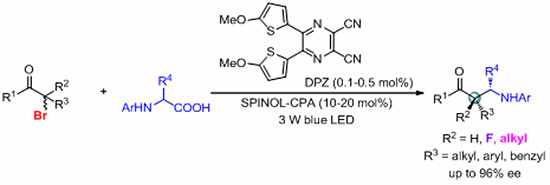
The scientific significance of this work lies in the successful breakthrough that the simple halogenated aromatics cannot obtain the corresponding optical pure products through the classic nucleophilic substitution reactions (SN1 and SN2), and provide a new synthetic route for the asymmetric synthesis of b-amino carbonyl compounds.
Dr. LI Jiangtao of class 2016 is the first author, and professor JIANG Zhiyong is the only corresponding author.
Full text link: https://www.nature.com/articles/s41467-018-04885-3.pdf.

 News /
Content
News /
Content


