Recently, Prof. Zhihong Tian, a Yellow River Scholar of Engineering Research Center for Nanomaterials, has collaborated with Dr. Qingran Zhang and Dr. Xunyu Lu of the University of New South Wales in Australia, and Prof. Markus Antonietti of the Max Planck Institute of Colloids and Interfaces in Germany in the field of electrochemical oxygen reduction and has reapened several important achievements; some of which were published in Angewandte Chemie International Edition with the title of "Constructing Interfacial Boron-Nitrogen Moieties in Turbostratic Carbon for Electrochemical Hydrogen Peroxide Production". (Angew. Chem. Int. Ed. 2022, e202206915)
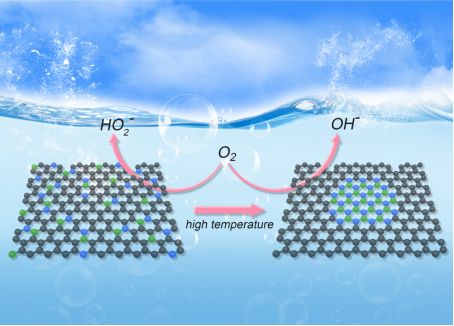
Hydrogen peroxide (H2O2) is an important chemical, and the traditional anthraquinone preparation method of which has the disadvantages of consuming a large amount of energy and spewing pollution and posing safety hazards. These drawbacks demand a greener alternative - Electrochemical oxygen reduction reactions. To catalyze oxygen reduction reactions, boron (B) and nitrogen (N) co-doped carbon are the necessary and adequate materials, but the design and synthesis of these catalysts are still extremely challenging.
In this study, a novel carbon catalyst was prepared using an ionic liquid-assisted doping strategy. The results of the study proved that the catalyst has a highly dispersed and uniformly distributed interfacial B-N-C structure, which tends to aggregate into the hexagonal boron nitride (h-BN) domains at higher pyrolysis temperatures. This particular finding provides a powerful chemical tool for investigating the "structure-catalytic selectivity" relationship of catalysts during electrochemical oxygen reduction. The catalyst has high H2O2 activity and selectivity, which offers new ideas for the efficient green preparation of H2O2 and also provides a reference for other possible electrochemical reactions.
This work was supported by the National Natural Science Foundation of China, the Science and Technology Department of Henan Province, Henan University, the University of New South Wales in Australia and the Max Planck Society in Germany.
The paper link: https://onlinelibrary.wiley.com/doi/10.1002/anie.202206915

 Research /
Research Achievements /
Science & Technology /
School of Chemistry and Molecular Sciences /
Content
Research /
Research Achievements /
Science & Technology /
School of Chemistry and Molecular Sciences /
Content


