Chem. Commun. 2020, 56, 1074-1077, DOI: 10.1039/c9cc08971c
Authors:Bingyue Li, Xiaojuan Zhu, Jianwei Wang, Ruimin Xing, Qian Liu, Xifeng Shi, Yonglan Luo,* Shanhu Liu,* Xiaobin Niu * and Xuping Sun
Chemical Synthesis and Pollution Control Key Laboratory of Sichuan Province, College of Chemistry and Chemical Engineering, China West Normal University, Nanchong 637002, Sichuan, China.
Henan Key Laboratory of Polyoxometalate Chemistry, Henan Engineering Research Center of Resource & Energy Recovery from Waste, College of Chemistry and Chemical Engineering, Henan University, Kaifeng, 475004, P. R. China
School of Materials and Energy, University of Electronic Science and Technology of China, Chengdu 611731, Sichuan, China.
E-mail:liushanhu@vip.henu.edu.cn, luoylcwnu@hotmail.com, xbniu@uestc.edu.cn;
As an essential activated nitrogen source, NH3 plays a vital role in both human life and the earth’s ecosystem. It is also regarded as a carbon-neutral energy carrier with high energy density for the hydrogen economy. However, due to the strong N N bond, it is difficult to mildly reduce N2 to NH3. Industrial-scale NH3 production occurs via the Haber–Bosch (H–B) process using N2 and high-purity H2 over Fe-based catalysts at high temperature and pressure, which demands 1–3% of the world energy supply and generates abundant CO2. As a promising substitute to the H–B process, electrochemical N2 reduction has aroused great attention which directly utilizes abundant water as the hydrogen source and can be readily coupled with an intermittent renewable energy source.
Recently, Liu’s group developed a novel Ti3+ self-doped TiO2-x nanowires on Ti mesh (Ti3+–TiO2-x/TM) as an efficient NRR electrocatalyst to convert N2 to NH3 with excellent selectivity. When tested in 0.1 M Na2SO4, this catalyst offers a high FE of 14.62% with a large NH3 yield of 3.51ⅹ10-11 mol s-1 cm-2 at -0.55 V vs. the reversible hydrogen electrode (RHE), superior to its TiO2/TM (6.49%; 1.89ⅹ10-11 mol s-1 cm-2). Meanwhile, it also shows high electrochemical stability. The mechanism for such enhancement is further studied by Density Functional Theory (DFT) calculation and can be attributed to the reaction energy barrier being greatly decreased and the active sites being increased by Ti3+ self-doping.
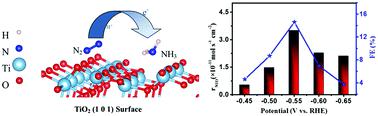
Figure 1. Table of Contents Entry for Ti3+ self-doped TiO2-x nanowires on Ti mesh
Bingyue Li, a master student of 2017, Xiaojuan Zhu, a master student of 2017, Jianwei Wang, a teacher, are the first authors with equal contribution.
Yonglan Luo, Shanhu Liu and Xiaobin Niu are the co-corresponding authors.
The result was jointly completed by Henan University, China West Normal University and University of Electronic Science and Technology of China.
This work was supported by the National Natural Science Foundation of China (No. 21575137)
LINK: https://doi.org/10.1039/C9CC08971C.
Chem. Commun.: SCI, top, IF= 6.16

 Research /
Research Achievements /
Science & Technology /
School of Chemistry and Molecular Sciences /
Content
Research /
Research Achievements /
Science & Technology /
School of Chemistry and Molecular Sciences /
Content


