Professor Lixin Zhang's team of the School of Life Sciences and Professor Sui Sen-fang's team of the Southern University of Science and Technology/Tsinghua University cooperatively published a research article of "Regulatory dynamics of higher plant PSI-LHCI supercomplex during state transitions" in Molecular Plant on November 7th, 2023. They proposed a novel regulatory mechanism of state transitions, a kind of light acclimation in higher plants.
In natural environment, the fluctuant light conditions affect the normal operation of photosynthesis, which induce the reduction of the photochemical efficiency of plants and even cause the light damage. To maintain the higher efficiency of photosynthesis, photosynthetic organisms have evolved several short-term and long-term acclimated mechanisms, which either enhance the light harvesting of plants under dim light condition or reduce the photodamage of photosynthetic machines under high light or fluctuant light conditions. Therefore, exploring the molecular mechanisms of light acclimation in higher plants under the changing light conditions is of great significance in both theoretical research and practical production.
State transition (ST) is an important light acclimated mechanism in plants. Upon the excitation of photosystem II (PSII) (e.g., blue light), light-harvesting complex II (LHCII) was phosphorylated, which dissociates from PSII and binds to photosystem I (PSI) (referred to as state 2, ST2). Upon excitation of PSI (e.g., far-red light), LHCII is dephosphorylated and moved back to PSII (referred to as state 1, ST1). Previous studies have reported the cryo-EM structures of PSI-LHCI-LHCII in maize and green algae in state 2 (Pan et al., 2018; Huang et al., 2021; Pan et al., 2021). However, it remains unclear whether the conformation of PSI-LHCI itself undergoes conformational changes during state transition to adapt the changing light conditions, as well as the dynamic binding and possible regulatory mechanisms of the binding of LHCII.

In this study, the Arabidopsis thaliana seedlings in state 1 and state 2 were induced with different light conditions, respectively. Then the related complexes were purified by native PAGE-Size exclusion chromatography, and the structures of PSI-LHCI-ST1 and PSI-LHCCI-LHCII-ST2 were resolved by the single particle analysis of Cryo-EM (Fig. 1). Based on structural analysis, further combined with genetic characterization and physiological verification, they found that PsaK, as a novel docking site, promotes the binding of LHCII trimer to the PSI core complex through the interactions with Lhcb1. Meanwhile, the conformational changes of PsaL/PsaK/PsaA subunits enhance the binding of PsaO to the PSI core in state 2, further stabilizing the binding of LHCII trimer (Fig. 2).
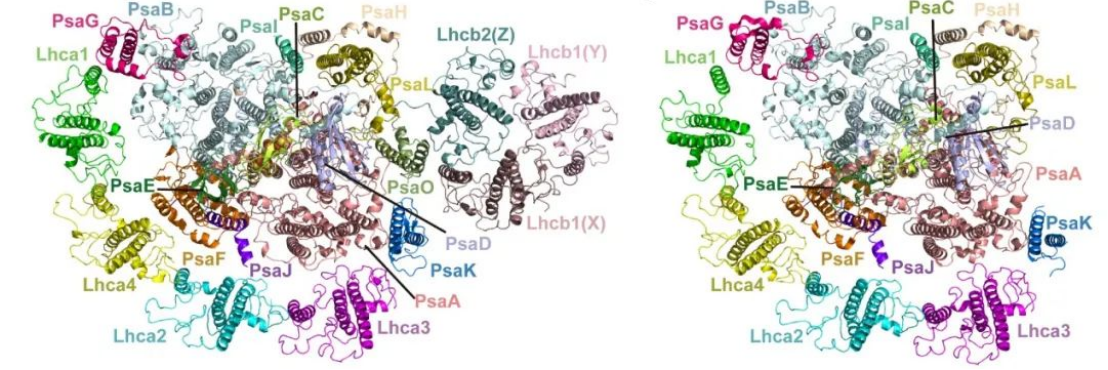
Fig. 1 Overall architectures of the PSI-LHCI-LHCII-ST2 (left) and PSI-LHCI-ST1(right) supercomplexes from Arabidopsis.
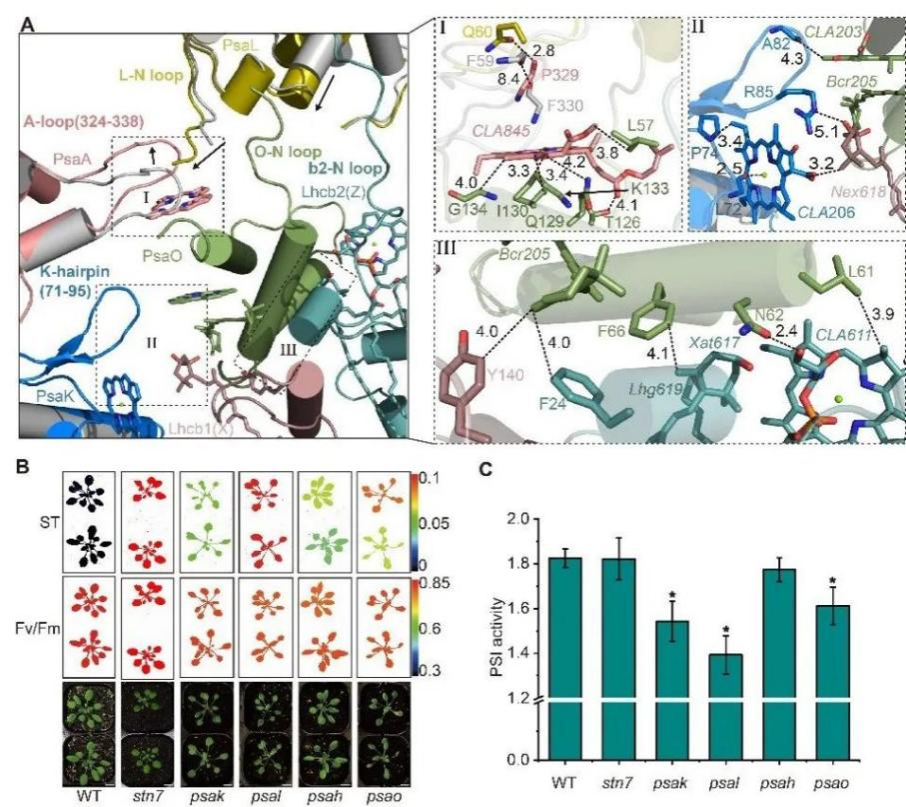
Fig. 2 Dynamic conformational changes of PsaL/PsaK/PsaA contribute to the assembly of PsaO and LHCII trimer. A. The conformational changes of the interviews between PsaL/PsaK/PsaA and PsaO/Lhcb2/Lhcb1 in PSI-LHCI-ST1 (gray) and PSI-LHCI-LHCII-ST2 (iridescence). B. Chlorophyll fluorescence analyses of Arabidopsis mutants. C. The analyses of PSI activity of Arabidopsis mutants.
Superposition of PSI-LHCI-ST1 and PSI-LHCI-LHCII-ST2 showed that PSI-LHCI module had a more compact conformation in state 2, and the Mg-to-Mg distance of the most chlorophyll pairs in LHCI was shorter, enhancing the interaction between LHCI and the PSI core, thereby improving the excitation energy transfer efficiency from the peripheral subunits to the PSI core. In summary, based on the cryo-EM structural models of PSI-LHCI in two states, combined with genetic characterization, physiological and biochemical analyses, this work revealed a novel model of PSI-LHCI supercomplex that involved in state transitions, and elaborated on the new mechanism of light acclimated regulation of higher plants, thus providing a theoretical basis for improving the light adaptation ability and improving varieties of crop.
Wu Jianghao, a postdoctoral fellow of the State Key Laboratory of Crop Stress Adaptation and Improvement/School of Life Sciences of Henan University, and Chen Shuaijiabin, a doctor of Southern University of Science and Technology/Tsinghua University, are the co-first authors of this paper. Professor Lixin Zhang from the State Key Laboratory of Crop Stress Adaptation and Improvement/School of Life Sciences of Henan University and Professor Sui Sen-fang from Southern University of Science and Technology/Tsinghua University are the co-corresponding authors. This research was funded by the National Key R&D Program and the National Natural Science Foundation of China.
Professor Lixin Zhang's team has been engaged in research on the regulation mechanism of plant photosynthesis for a long time and has achieved a series of innovative and systematic research results. In recent years, the research team has published a number of high-level research papers in international journals such as Cell and undertaken national scientific research projects, such as the National Key R&D Program and the Key Projects of the National Natural Science Foundation Joint Fund of China. These achievements enrich our understanding of the mechanism of photosynthesis and make an important contribution to the development of photosynthesis research. Meanwhile, these findings provide an important theoretical basis for achieving efficient utilization of light energy in crops and improving photosynthesis efficiency. They have significant application values for crop high-light efficiency breeding.



