Photosynthesis is the largest use of solar energy on the earth, the process of synthesis of carbon dioxide into organic matter, and the release of oxygen. Chloroplast is the place of photosynthesis. Chloroplasts are evolved from the symbiosis of photosynthetic bacteria and play a key role in photosynthesis and other important physiological processes. Chloroplast is semi autonomous organelle and 95% of chloroplast proteins are encoded by nuclear genes. After they are synthesized into precursors in the cytoplasm, the nuclear-endoded protein are imported into chloroplasts. However, one of the remarkable characteristics of chloroplast is its highly complex structure, with multiple subcellular membranes (outer membrane, inner membrane and thylakoid membrane), thus depicting three independent compartments (inter membrane space, matrix and inner cavity). Therefore, it is very challenging to understand the molecular mechanism of chloroplast protein targeting and sorting.
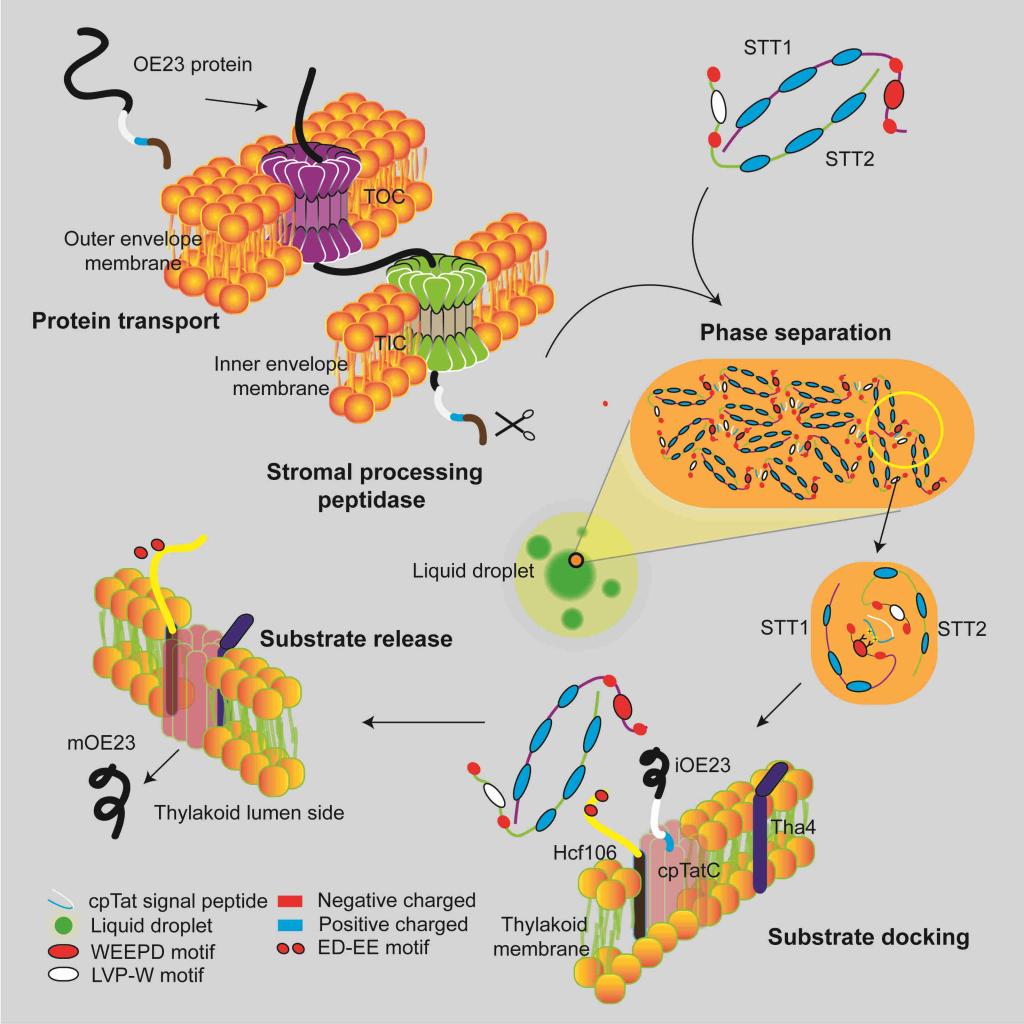
In this study, STT1 and STT2, the key protein transport sorting factors in the chloroplast stroma, were identified, and the molecular mechanism of substrate recognition, sorting and transport targeting of cpTat dependent transport pathway was elucidated. Both STT1 and STT2 proteins contain an IDR (internally separated region) domain at the N-terminal and an ankyrin repeat domain at the C-terminal. STT1 and STT2 can form an elliptical spherical heterodimer structure depending on the interaction of ankyrin repeat domain. The IDR region of STT1 and STT2 contains conserved WEEPD motif and LPV-W motif respectively, which is responsible for interaction with the twin-arginine motif and hydrophobic domain of substrate signal peptide. The results showed that the substrate can activate the STT complex to further assemble the phase separation to form concentrated droplets. STT substrate phase separation droplets assist the substrate to pass through the chloroplast stroma to target the thylakoid membrane. Hcf106 can inhibit the phase separation of STT and release the substrate, and complete the proper transport and assembly of the substrate. This may not only ensure the substrate folding, but also keep the activity of the substrate signal peptide for the downstream TatC/HCF106 receptor recognition. Blocking the binding of STT-HCF106 will block the transport of Tat substrate and affect the photosynthesis of plants, leading to the lethal phenotype of plants. In this study, it was found for the first time that phase separation regulates chloroplast protein transport, thus regulating chloroplast biogenesis and photosynthesis. This study reveals the conserved mechanism of regulating the separation of organelle biogenic protein transporters, and opens up a new way to study how cells precisely regulate physiological processes.
The research work is supported by NSFC, Ministry of science and technology, Henan University and School of life sciences.
DOI: 10.1016/J.Cell. 2020.02.045
Paper link: https://www.cell.com/cell/fulltext/S0092-8674(20)30222-1



