Glioblastoma (GBM) is the most common central nervous system brain tumor, with a high incidence and mortality rate at home and abroad. However, there are a lack of clinical trials that are in safe and effective treatment modalities. Furthermore, GBM is difficult to treat due to the complex tumor microenvironment, the low efficacy of monotherapy, and the high possibility of drug resistance. To address the aforementioned challenges, the laboratory has designed arsenic-based nanomedicine to regulate tumor microenvironment, as well as other novel therapeutic strategies based on metal chelation therapy, gene chemical dynamic combination therapy, and CRISPR/Cas9 gene editing. This development resulted in beneficial outcomes.
Arsenic-based nanomaterial for GBM
Considering the redox and hypoxia properties in tumor microenvironment, the lab designed an emerging nanomedicine using silver nanoparticle with chelating As(V) ions. This nanomedicine displayed the features of glutathione (GSH) stimulus responsive ability and endosomal escape. The GPx and SOD enzymes' activity can be specifically inhibited by nanomedicine in vivo, which triggered LC3-mediated autophagy. Results from both in vitro and in vivo experiments support the use of As-based chemotherapy, opening the path for effective boronate affinity-based arsenic chemotherapeutics enabled by nanomedicine for on-demand site-specific cancer combination treatment of GBM tumors. The research paper was published in ACS Applied Materials & Interfaces 2022, 14, 32, 36487–36502 (IF=10.383). Ph.D. candidate Jiliang Yan and Dr. Sumaira Hanif are the first authors, and Dr. Haigang Wu and Dr. Pir Muhammad are co-corresponding authors.
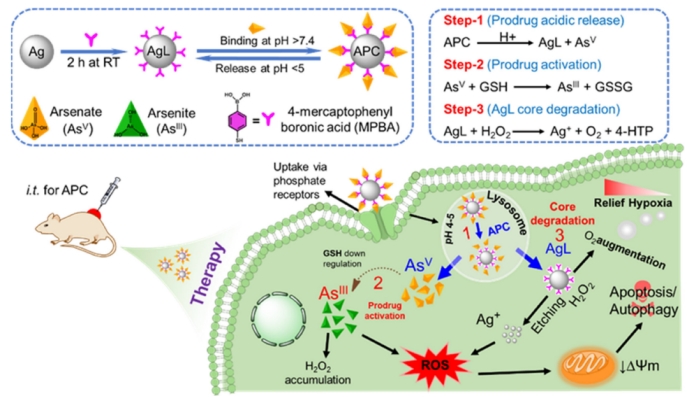
Paper link: https://doi.org/10.1021/acsami.2c12076
Metal chelation therapy for GBM
The copper (Cu) chelator, di-2-pyridylketone-4,4-dimethyl-3-thiosemicarbazone (Dp44mT) attracted much interest due to its potent anti-tumor activity mediated by the formation of a highly redox-active Cu-Dp44mT complex. However, its translational potential was limited by the development of toxicity in animal models reflecting poor selectivity. The laboratory overcame the limitations of Dp44mT by incorporating it in new Angiopep-2 decorated biomimetic nanoparticles co-loaded with the BBB regulator Regadenoson to improve its blood-brain barrier (BBB)-penetration and cancer selectivity. The present study also uniquely employed CuII-ATSM to develop a Cu-enriched U87MG tumor model designed to enhance the therapeutic effect of Dp44mT mediated by Cu chelation, ROS and apoptosis induction. The nanomedicine effectively inhibited tumor growth and prolonged the survival of the tumor-bearing mice without evident side effects.
The research paper was published in Biomaterials 2022, 289, 121760 (IF=15.305). Dr. Muhammad Ismail, Ph.D. candidate Wen Yang and M.D. student Yanfei Li are the first authors, and Prof. Shi and Prof. David B. Lovejoy are the co-corresponding authors.
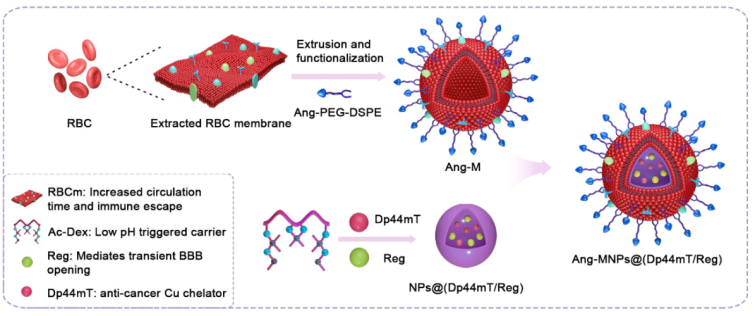
Paper link: https://doi.org/10.1016/j.biomaterials.2022.121760
Chemokinetic and RNAi combination therapy for GBM
Taking advantage of the non-invasive and high response characteristics of chemodynamic therapy (CDT), a biomimetic nanomedicine was developed with metastatic melanoma cell membrane as shell, charge-converted citraconic anhydride grafted poly-lysine (PLL-CA) as middle layer and siRNA-complexed polyethyleneimine xanthate (PEX) as the core. The developed biomimetic nanomedicines can effectively penetrate BBB. Interestingly, PEX chelates the abundant copper ions in tumor cells, resulting in GSH depletion and producing •OH via Fenton-like reaction to kill tumor cells. Additionally, the gene silencing of anti-apoptotic protein Bcl-2 leads to the increase of ROS level, which induces the apoptosis cascade of GBM cells. Excellent therapeutic effect and prolonged survival were achieved in both subcutaneous melanoma and orthotopic GBM tumor models.
The research paper was published in Advanced Functional Materials 2022, 2209239 (IF=19.925). Ph.D. candidate Dongya Zhang is the first author, and Prof. Shi, Prof. Zheng and A/Prof. Zou are the co-corresponding authors.

Paper link: https://doi.org/10.1002/adfm.202209239
CRISPR/Cas9 gene editing therapy for GBM
Utilizing the high specificity and efficiency of CRISPR gene editing technology, the lab developed a new polymer nanocarrier, which can significantly improve the encapsulation and loading of Cas9 RNP components by modifying guanidine and fluoro groups. The prepared nanoparticles can cross blood-brain barriers (BBB) with ease, demonstrating superior blood circulation time, cellular uptake, and drug loading efficiency. Furthermore, they are biocompatible and efficiently edit oncogenic genes, ultimately inhibiting tumor growth. Thus, the polymer nanocarriers have a promising prospect in clinical translation for treating GBM.
The research paper was published in the Journal of Controlled Release 2022, 351, 739–751 (IF=11.467). A/Prof. Ruan, M.D. student Mingzhu Jiao and Ph.D. candidate Sen Xu are the first authors, and Prof. Shi, Prof. Zheng and Director Rongjun Qian from Henan Provincial People's Hospital are the co-corresponding authors.
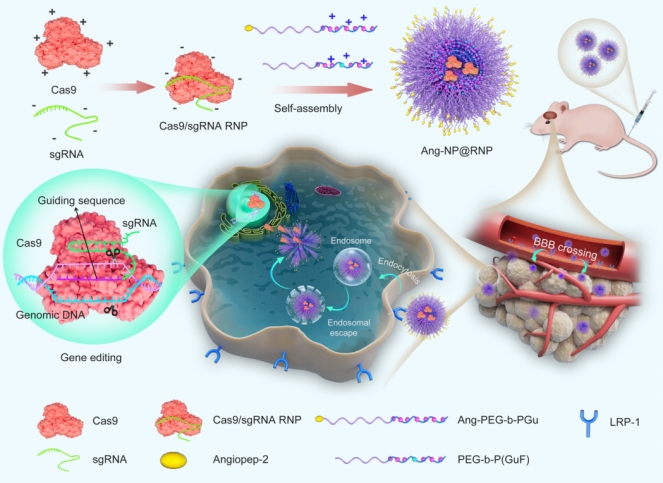
Paper link: https://doi.org/10.1016/j.jconrel.2022.09.046
Laboratory Website: https://bs.henu.edu.cn/cn.htm

 News /
Content
News /
Content


